Abstract
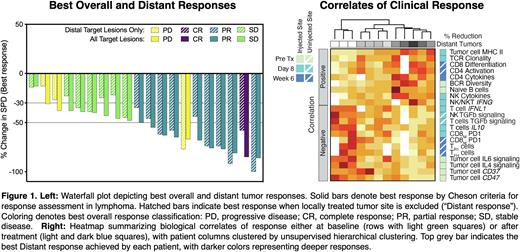
In situ vaccination (ISV) is an immunotherapeutic strategy where immunostimulatory agents are directly injected into a tumor lesion to trigger an immune response to tumor antigens locally that can then migrate to attack the tumor at sites throughout the body. Here we report clinical and immunological results of a phase I/II trial in non-Hodgkin Lymphoma (NCT02927964) in which we combined ISV using intratumoral SD101 (a TLR9 agonist) and low-dose radiation (2 x 2 Gy), and daily oral ibrutinib, a kinase inhibitor that modulates both B and T cells. Twenty patients were evaluable for safety and efficacy. Most had follicular lymphoma, and all were previously treated with 1-5 prior lines of therapy. Adverse events were primarily low grade and included flu-like reactions after intratumoral injections, and fatigue, diarrhea, and rash. The overall tumor response rate was 50%, including one patient with a complete response (Figure 1, left). All patients experienced tumor reduction at distant (non-injected, non-irradiated) index lesions (median reduction 45%, range 13-100%), indicative of systemic immune responses. To understand the dynamic immune modulation induced by our treatment strategy, we obtained serial fine needle aspirates from injected and uninjected tumor sites at screening (Pre Tx) and during treatment (Day 8 and Week 6). In-depth single cell analyses revealed baseline predictors of response, treatment-induced effects, and treatment-induced correlates of clinical response. Baseline tumor cell expression of CD47, CD37, and IL4 and IL6 signaling-related transcripts portended lesser clinical responses, while treatment-induced antigen presentation by tumor cells, activation of NK and T cells, and expansion of T cell clones in the tumor all associated with deeper clinical responses (Figure 1, right). Notably, our treatment dampened immune suppressors such as TGFß signaling, inhibitory T regulatory 1 cells, and CD8 T cell PD1 expression. These reductions correlated positively with clinical outcomes, suggesting that reversal of negative regulators was as important as induction of immune effectors in producing robust immune responses and clinically meaningful tumor responses.
Disclosures
Shree:Gilead Sciences: Other: Spouse's employment. Frank:Allogene Therapeutics: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Research Funding; Kite/Gilead: Honoraria, Research Funding; Roche/Genentech - Wife: Current equity holder in private company, Current holder of stock options in a privately-held company. Alizadeh:Foresight Diagnostics: Consultancy, Current equity holder in private company, Patents & Royalties; Cibermed Inc: Consultancy, Current equity holder in private company, Patents & Royalties; Adaptive Biotechnologies: Consultancy; Gilead: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties; Syncopation: Current equity holder in private company, Patents & Royalties; Roche: Consultancy; BMS: Consultancy, Research Funding; Genentech: Consultancy; Karyopharm: Consultancy. Advani:ADC Therapeutics, BMS, Daiichi Sankyo, Epizyme, Gilead, Incyte, Merck, Roche, Sanofi: Consultancy; ADC Therapeutics, Cyteir, Daiichi Sankyo, Gilead, Merck, Regeneron, Roche, Seattle Genetics: Research Funding. Long:Lunit: Consultancy. Khodadoust:Nutcracker Therapeutics: Research Funding; Myeloid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Research Funding. Levy:Quadriga BioSciences: Consultancy; BeiGene: Consultancy; GigaGen: Consultancy; Teneobio: Consultancy; Nurix: Consultancy; Dragonfly: Consultancy; Apexigen: Consultancy; Viracta Therapeutics: Consultancy; Spotlight Therapeutics: Consultancy; Immunocore: Consultancy; Walking Fish Therapeutics: Consultancy; Kira Pharmaceuticals: Consultancy; Abintus Bio: Consultancy; Khloris Biosciences: Consultancy; Virsti Therapeutics: Consultancy; BioLineRx: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.